Current Activities
Early Career Best Presentation Award
The EFC Task Torce "Corrosion of Medical Implants and Devices" is pleased to announce that its first "Early Career Best Presentation Award"
was presented during EUROCORR 2025 by Dr. Patrik Schmutz (Task Force Chair) to
Stefan Reinelt
from University of Freiburg, IMTEK.

The award was kindly sponsored by "Straumann group".

Business Meeting
Task Force business meeting at EUROCORR 2026
Online seminar series
A series of online meetings for the Task Force members with a stronger involvement/contribution of clinicians and biologists has been initiated. The presentations focus on clinical and biological aspects of the implant/biology interface.
Interested in these webinar… register to the Task Force and you will receive the information and meeting links in advance.
Publications
Nature portfolio – npj Materials Degradation special issue on
- Corrosion of Medical Implants and Devices
- Link to the special issue: Corrosion of Medical Implants and Devices
- New submission deadline: 31 December 2025
This Collection welcomes perspectives and original research articles and review papers [pas1] dealing with temporary (Mg, Zn, Fe, or Mo alloys) and permanent (Ti, Stainless Steel, Co-Cr-Mo alloys, Pt and others) metallic medical devices and implants including but not limited to the following topics:
- Development of new metallic materials and processing routes (including additive manufacturing) with improved degradation profile, functional surface coatings, durability and mechanical properties
- In vitro – in vivo correlation for improving testing protocols and predicting implant service life also in terms of particle and ionic leaching
- Degradation mechanisms and identification of important influencing factors
- Shaping the degradation profile of temporary implants with protective and drug releasing coatings
- Computational modeling of implant degradation and lifetime prediction
INvolvement
For those who are interested in the subject and wish to keep in contact with the Task Force to learn about our activities, click here to register.
Mission and objectives
Mission
The interactions of medical materials for implants and devices with biological matter are still largely unknown and their potentially detrimental effect on the material stability are sometimes clearly underestimated, evident from a raising number of failure reports. Nevertheless, the medical field relies more and more on the use of metallic materials for various therapies ranging from implants for fracture fixation or cardiovascular intervention, including those with drug-delivery capabilities, to increasingly advanced medical devices including those for monitoring and sensing. Consequently, reliable testing protocols are required that reflect the dynamic, complex and aggressive local chemical environment that biological fluids and tissues create (e.g. large pH variation in wound and inflammation conditions). Additionally, these testing protocols need to provide the required level of detail, i.e. at relevant sensitivity and magnification at which material dissolution may occur.
In fact, considering the current understanding of the material-biology interface interactions, it is evident that macroscopic aspects of implant/medical device failure, such as fractured implants or material particle release, are already documented. There is, however, a clear lack of understanding of the processes occurring that underlie the formation of micro- and nanoscale corrosion products and ionic leaching, as well as their consequences.
OBJECTIVES
The new Task Force (TF) activities aim at a better understanding of these "invisible" processes for a better biocorrosion prediction and ultimately prevention of implant & medical device failure (see Figure). The TF thus addresses patient safety, with the patient as the prime stakeholder. The work of the TF on "corrosion of medical implants and devices" focusses on the following fundamental and practical topics with an "understand-predict-prevent" approach:
- improving in vitro protocols for corrosion susceptibility assessment of permanent and biodegradable implants/devices
- identifying mechanisms underlying the formation of nanoscale, molecular and ionic products and deriving structure-stability/reactivity correlations. This understanding will support development of corrosion prevention strategies
- developing new analytical methodologies to advance the detection limit (trace element, lateral resolution) for more detailed investigation of degradation mechanisms.
The TF will foster collaboration and exchange between various expert fields from material science, electrochemistry to biology, with their fundamental knowledge and experimental capabilities. We will increase awareness for chemical surface reactivity and enhance visibility of the topic to attract biomaterials expert and clinicians to foster the discussion through their input on clinical challenges and patient needs. The TF actions aim at supporting a responsible behavior in the development of improved medical technology.
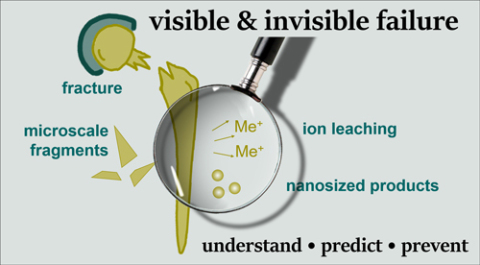
Figure: Task Force focus towards extending the current understanding of biocorrosion mechanism (design: courtesy of Martina Cihova)
What is urgently needed, especially from a patient health perspective, is better tracking (detection) and tracing (distribution within the human body) of released particles, molecular and ionic species. The prime focus of the TF is on metallic implants/devices but not restricted to them, acknowledging that polymer degradation can also pose significant problems. For either material class, processes occurring at the nanoscale are often overlooked according to 'what cannot be seen does not exist' and, consequently, their potential toxicity or other adverse biological effects may go unassessed.
In the corrosion mechanisms description, the TF will work on experimental protocols integrating chemical stability criteria in implant/device validation processes, considering also worst-case scenarios. For instance, it should be considered that most implanted objects will experience some degree of micromotion and corrosion-fatigue, making simple immersion tests insufficient for validation. The TF aims at defining improved testing protocols that also include not yet documented mechanical loading situations.
Online Seminars
Next seminar to come
28th October 2025
Prof. Yolanda Hedberg
Western Science,
London Ontario, Canada
Title: Biological and patient factors influencing corrosion reactions of biomedical implant materials
Previous seminars
21st January 2025
Asst. Prof. Rihard Trebše, M.D.Ph.D.
Consultant Orthopedic surgeon
Head of Service for Bone infections
Valdoltra Orthopedic Hospital, Slovenia
Title: “Corrosion related orthopedic device failures”
17th September 2024
Dr. Heinz Müller
Fellow New Technologies, Innoventures Program,
CORTRONIK GmbH, Rostock-Warnemünde, Germany
Title: “Magnesium-Based Vascular Implants – Technology, Degradation and Testing”
18th June 2024
Prof. Ingrid Milosev
Head of the Department of Physical and Organic Chemistry,
University of Ljubljana, Slovenia
Title: "Implant failure understanding at the Vladoltra Orthopaedic Hospital (Slovenia): the approach, long experience and database"
Previous activities
EUROCORR 2025 in Stavanger
- TF symposium:
Corrosion prediction for medical implants and devices
Chairs: Dr. Schmutz, Dr. Lamaka, Dr. Cihova
- Joint Symposium (TF + TF Additive Manufacturing):
Degradation of orthopaedic joint implant Corrosion and corrosion protection of additive manufactured metals for biomedical applications
Chairs: Prof. Iris De Graeve, Dr. Patrik Schmutz, Dr. Reynier Revilla, Dr. Sviatlana Lamaka
EUROCORR 2024
- TF Session: Corrosion prediction for medical implants and devices
Chairs: Dr. Schmutz, Dr. Lamaka, Dr. Cihova
- Joint Session (TF+ WP18): Degradation of orthopaedic joint implants
Chairs: Dr. Igual Munoz, Dr. Ripoll Dr. Schmutz
EUROCORR 2023
- TF Session: Corrosion of Medical Implants and Devices
Chairs: Dr. Patrik Schmutz, Dr. Manel Ripoll, Dr. Martha Alves, Dr. Beno Ter-Ovanessian
- Joint Session: Corrosion and corrosion protection of additive manufactured metals for biomedical applications
Chairs: Prof. Iris De Graeve, Dr. Patrik Schmutz
EUROCORR 2022
Workshop (TF was officialised at the STAC meeting 2022): "Corrosion of medical implants and devices"
EUROCORR 2021
Workshop (TF was created later in 2022): "Corrosion of medical implants and devices"